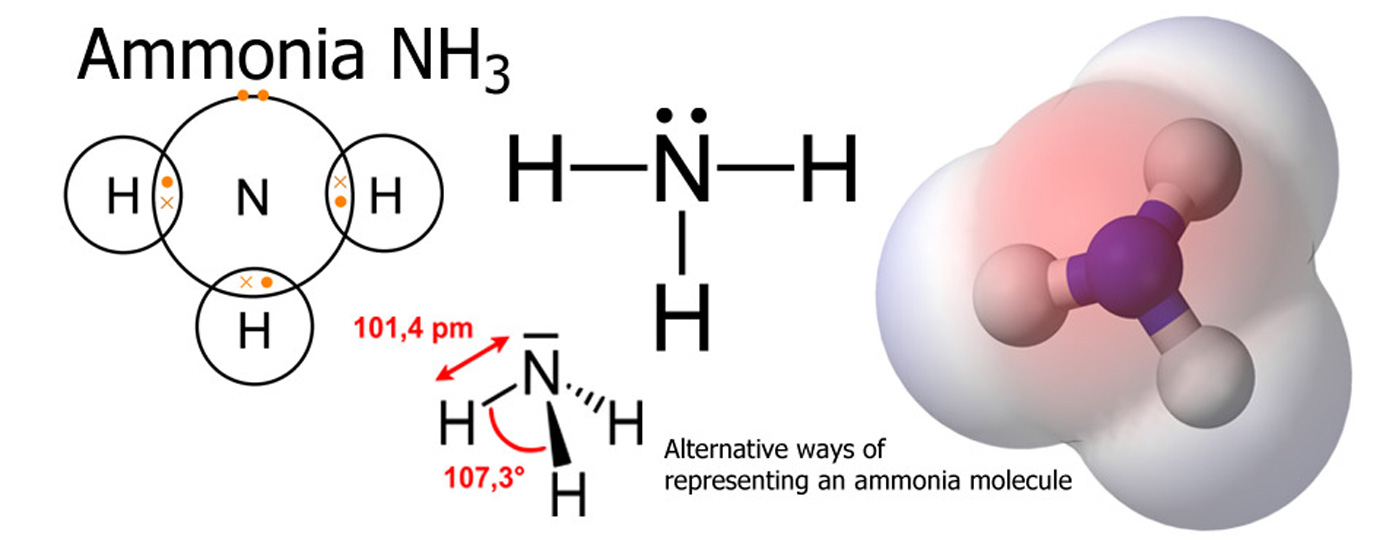
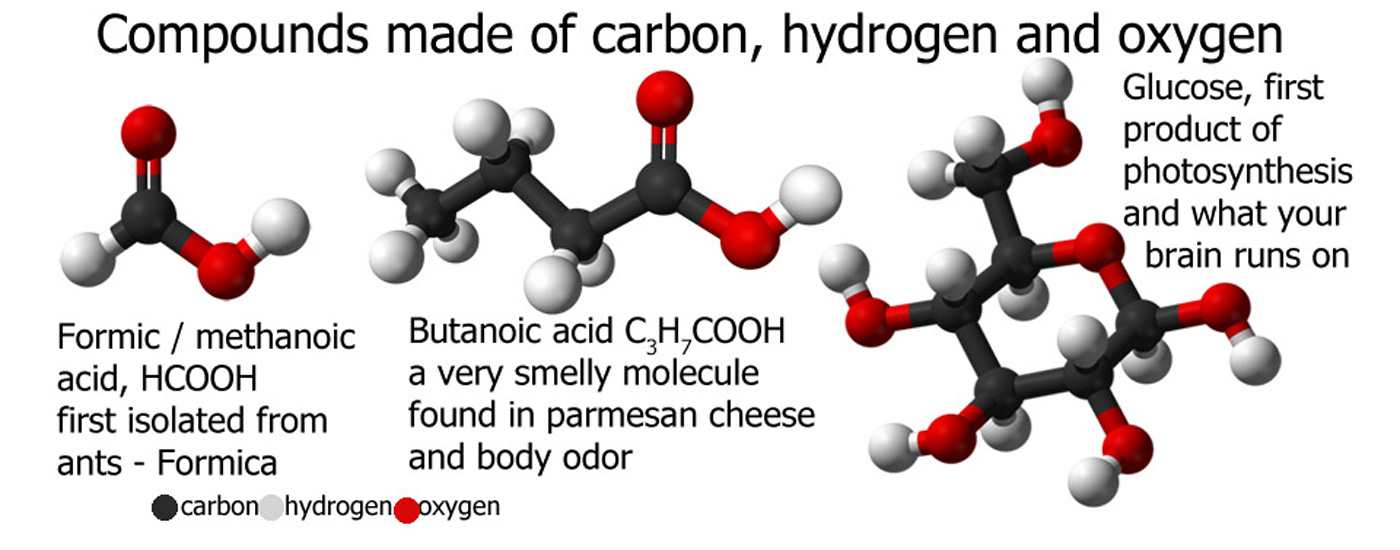
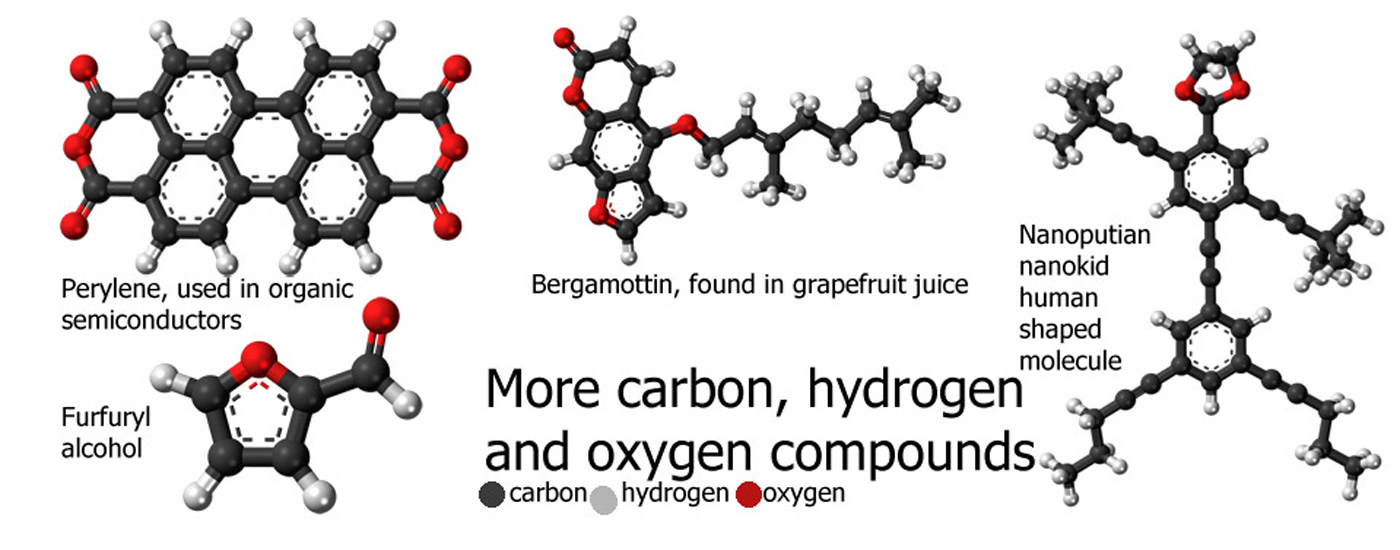
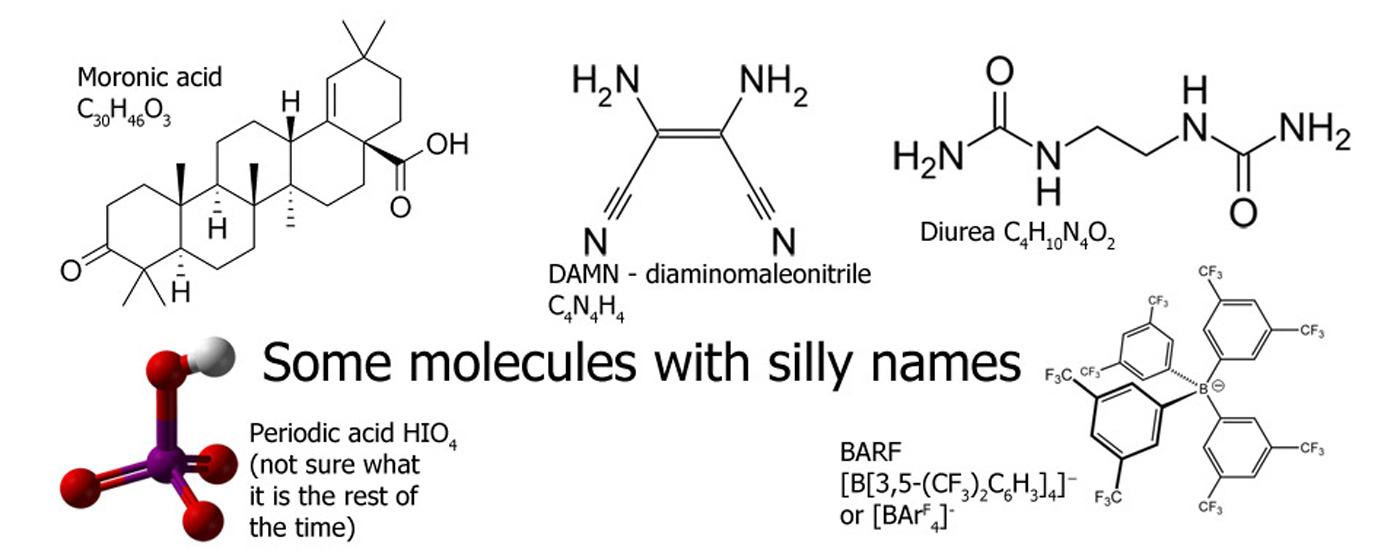
Chemical Compounds
Molecules
made of more than one kind of element are called compounds
A compound is formed when one kind of atom (an element) joins to at least one other kind of atom of a different element. The simplest compounds therefore just have two elements joined by a chemical bond, carbon monoxide, CO and common salt, sodium chloride NaCl are examples of simple compounds. There are many thousands of different compounds, simple, complex and everywhere inbetween.
Compounds usually have totally different
properties to the
elements that make them up.
Simple Compounds
CO - Carbon monoxide
Carbon - a black solid, it is the main
ingredient in charcoal and coal, it is unusual as it is a non-metal
that conducts electricity very well.
Oxygen
- a colourless, odourless gas, it is vital for the life of
many organisms, without it we will die in about five minutes.
Carbon monoxide - a colourless, odourless, deadly poisonous gas that can kill at concentrations of 0.1%.
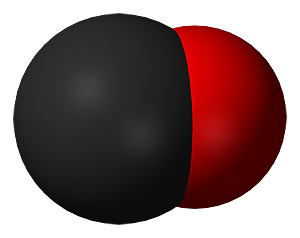 Carbon
monoxide - CO
Carbon
monoxide - COone carbon atom, one oxygen atom (mono - one)
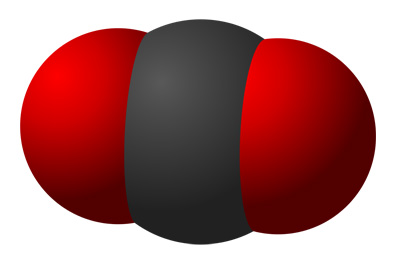 Carbon
dioxide - CO2
Carbon
dioxide - CO2one carbon atom, two oxygen atoms (di - two)
Space filling models - carbon in black, oxygen in red
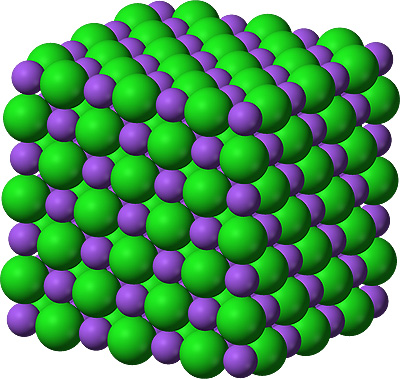
NaCl - Sodium chloride
Sodium - a soft metal that reacts violently
with water to make a caustic solution that will dissolve flesh.
Chlorine - a pale green, highly poisonous
gas,
that has been used directly as a chemical weapon.
Sodium chloride - common salt. A white crystalline solid that is essential for all animal life.
Compounds usually have totally different properties to the elements that make them up. It is not usually possible to guess the properties of a compound from its elements.
There are rules about what elements you can put together and in what numbers to make compounds. You can't just make up any old combination and hope it works any more than you can arrange letters together any way you like and expect to get a proper word.
Space filling models - sodium in purple, chlorine in green, carbon in black, oxygen in red
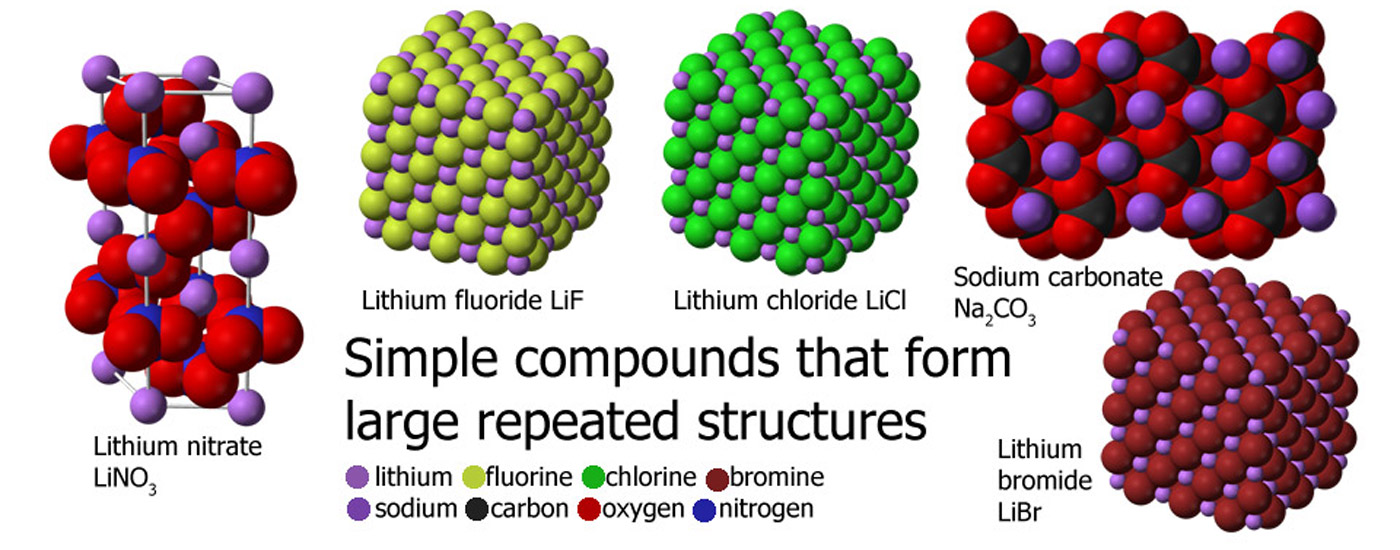
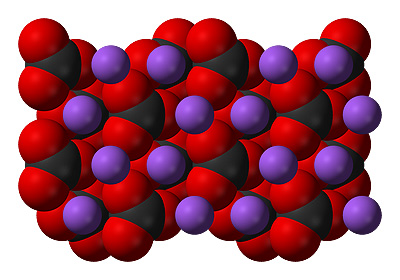
Like sodium chloride, sodium carbonate molecules like to hang around together and so form this sort of shape which makes a crystal.
Slightly more complicated compounds
Most compounds have more than two elements and have more than one of some or all of the elements. There is a little dropped number following the symbol of the element there is more than one of, such as:
CO2 - Carbon dioxide, 1 carbon atom and 2 oxygen atoms, 3 in total.
CH4 - Methane, 1 carbon atom and 4 hydrogen atoms, 5 in total.
LiNO3 - Lithium nitrate, 1 lithium atom, 1 nitrogen atom and 3 oxygen atoms, 5 in total.
Na2CO3 - Sodium carbonate, 2 sodium atoms, 1 carbon atom and 3 oxygen atoms, 6 in total.
Bigger more complicated compounds
Compounds range in size from the minimum number of two atoms to much larger structures with tens, hundreds or even thousands of atoms, here are a few of the larger ones:
Fe3Al2(SiO4)3 - Iron, aluminium silicate or almadine a form of the semi-precious stone garnet. 2 iron atoms, 2 aluminium atoms and then 3 lots of 1 silicon atom and 4 oxygen atoms, 3 + 2 + 3 x (1 + 4) = 20 atoms in total.
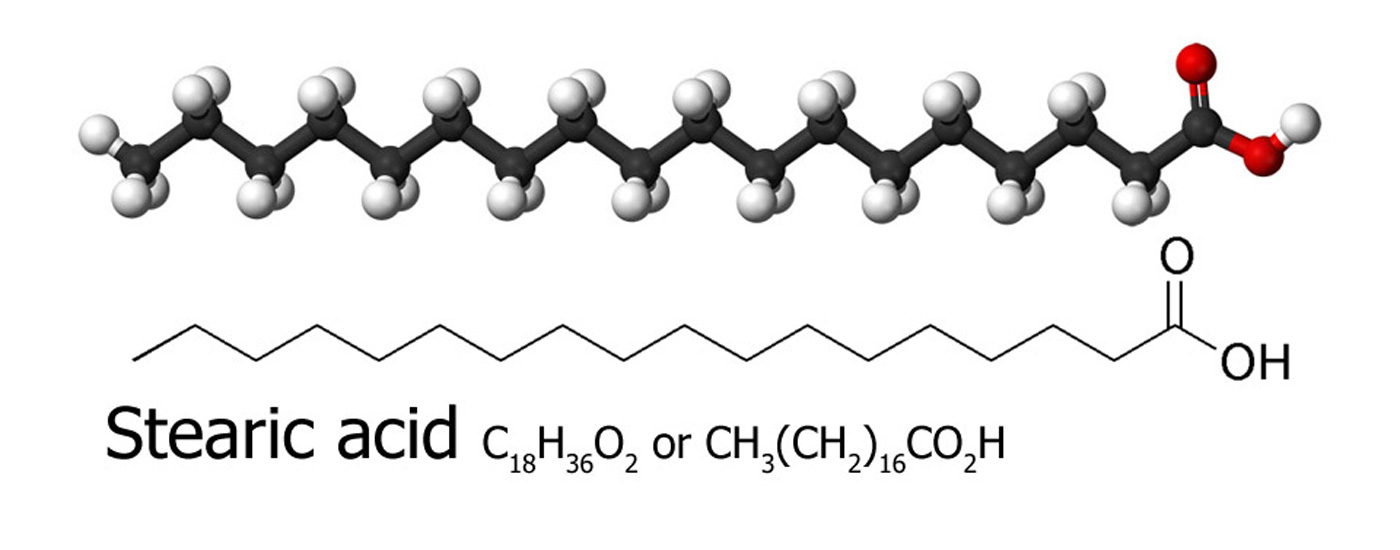
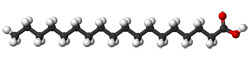 C18H36O2
- Stearic acid, a commonly found molecule made
by a variety of plants and animals. 18 carbon atoms, 36 hydrogen
atoms and 2 oxygen atoms, that's 56 in total.
C18H36O2
- Stearic acid, a commonly found molecule made
by a variety of plants and animals. 18 carbon atoms, 36 hydrogen
atoms and 2 oxygen atoms, that's 56 in total.
C256H381N65O76S6 - Insulin (from a pig in this case), a hormone that helps us to control our blood glucose levels, if we lose the ability to control glucose it results in diabetes. 784 atoms in total, I'll let you work out how it's made up from the carbon, hydrogen, nitrogen, oxygen and sulfur, you should have the hang of it by now.